Research:
ENDOCRINE RESISTANCE
Estrogen receptor alpha (ER, ESR1) is expressed in the majority of breast cancers and is a major regulator of breast cancer development and progression. Anti-endocrine therapy is one of the most efficacious and least toxic treatments for ER-positive (ER+) breast cancer (Nasrazadani et al., 2018). However, resistance is a major clinical problem and the majority of deaths from breast cancer are due to advanced endocrine resistant disease.
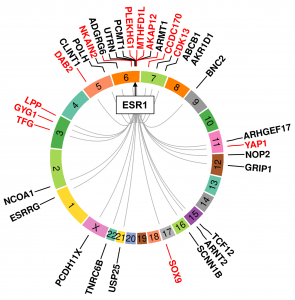
A recent and exciting development in the study of endocrine resistance has been the identification of mutations in ESR1, and our lab has focused on these alterations. We currently examine three different types of mutations, namely base pair mutations, fusions and amplifications. We reported on ESR1 mutations in circulating tumor DNA (Gyanchandani et al., 2017; Wang et al., 2016), showed that ESR1 mutations causes IGF pathway activation (Li et al., 2018) and provided the first description of context- and mutation site-dependent effects of the ESR1 mutations in genome-edited breast cancer cells (Bahreini et al., 2017). We were recently the first to report ESR1 fusion genes as a recurrent mechanism of anti-estrogen resistance (Hartmaier et al., 2018). Finally, we have reported on ESR1 amplification in advanced breast cancer (Basudan et al., 2019; Cao et al., 2019).
Current ongoing studies are examining the prevalence and activity of ESR1 alterations using numerous approaches including measurement in patient specimens by liquid biopsy (whole genome and capture sequencing) and rapid autopsy as well as analysis of function using naturally occurring and genome edited patient-derived organoids and xenografts.
Invasive Lobular Breast Cancer | Breast Cancer Metastasis | Precision Medicine
Bahreini, A., Li, Z., Wang, P., Levine, K. M., Tasdemir, N., Cao, L., Weir, H. M., Puhalla, S. L., Davidson, N. E., Stern, A. M., et al. (2017). Mutation site and context dependent effects of ESR1 mutation in genome-edited breast cancer cell models. Breast Cancer Res 19, 60.
Basudan, A., Priedigkeit, N., Hartmaier, R. J., Sokol, E. S., Bahreini, A., Watters, R. J., Boisen, M. M., Bhargava, R., Weiss, K. R., Karsten, M. M., et al. (2019). Frequent ESR1 and CDK Pathway Copy-Number Alterations in Metastatic Breast Cancer. Mol Cancer Res 17, 457-468.
Cao, L., Basudan, A., Sikora, M. J., Bahreini, A., Tasdemir, N., Levine, K. M., Jankowitz, R. C., McAuliffe, P. F., Dabbs, D., Haupt, S., et al. (2019). Frequent amplifications of ESR1, ERBB2 and MDM4 in primary invasive lobular breast carcinoma. Cancer Lett 461, 21-30.
Gyanchandani, R., Kota, K. J., Jonnalagadda, A. R., Minteer, T., Knapick, B. A., Oesterreich, S., Brufsky, A. M., Lee, A. V., and Puhalla, S. L. (2017). Detection of ESR1 mutations in circulating cell-free DNA from patients with metastatic breast cancer treated with palbociclib and letrozole. Oncotarget 8, 66901-66911.
Hartmaier, R. J., Trabucco, S. E., Priedigkeit, N., Chung, J. H., Parachoniak, C. A., Vanden Borre, P., Morley, S., Rosenzweig, M., Gay, L. M., Goldberg, M. E., et al. (2018). Recurrent hyperactive ESR1 fusion proteins in endocrine therapy-resistant breast cancer. Ann Oncol 29, 872-880.
Li, Z., Levine, K. M., Bahreini, A., Wang, P., Chu, D., Park, B. H., Oesterreich, S., and Lee, A. V. (2018). Upregulation of IRS1 Enhances IGF1 Response in Y537S and D538G ESR1 Mutant Breast Cancer Cells. Endocrinology 159, 285-296.
Nasrazadani, A., Thomas, R. A., Oesterreich, S., and Lee, A. V. (2018). Precision Medicine in Hormone Receptor-Positive Breast Cancer. Front Oncol 8, 144.
Wang, P., Bahreini, A., Gyanchandani, R., Lucas, P. C., Hartmaier, R. J., Watters, R. J., Jonnalagadda, A. R., Trejo Bittar, H. E., Berg, A., Hamilton, R. L., et al. (2016). Sensitive Detection of Mono- and Polyclonal ESR1 Mutations in Primary Tumors, Metastatic Lesions, and Cell-Free DNA of Breast Cancer Patients. Clin Cancer Res 22, 1130-1137.

